Back
Critical Care
Category: Quickshot Oral Session 18
Quickshot Oral : Quickshot Oral Session 18
SMART PREDICTS DICHOTOMOUS TOCILIZUMAB SURVIVAL EFFECTS IN HOSPITALIZED COVID-19 COVACTA PATIENTS
Monday, February 13, 2023
7:00am – 8:00am East Coast USA Time

- BO
BISMARCK OSUMO, MD
RESIDENT
INSPIRA HEALTH , United States - GS
Gus Slotman, MD
Inspira Health Network, United States
Presenter(s)
Principal Contact(s)
Objectives:
Background: Tocilizumab did not improve COVID-19 survival in the COVACTA randomized clinical trial (RCT). Clinical RCT entry criteria enroll so many study drug non-responders that treatment effects are diluted. This may have affected COVACTA. The SMART methodology identifies drug-responsive patients within RCT’s
Objective: To identify COVACTA patients among whom tocilizumab reduced COVID-19 mortality
Methods: On de-identified data from 438 COVACTA patients (144 placebo, 294 tocilizumab) pre-randomization stepwise logistic regression survival models were built separately for placebo and tocilizumab within three cohorts: 1. All COVACTA patients 2. COVACTA patients receiving steroids before/during the study. 3. No steroids. Pre-randomization data from all patients were entered into both models. Model interactions with 28-day survival determined optimum cutoffs, incrementally excluding from efficacy analysis patients predicted to be tocilizumab non-responders
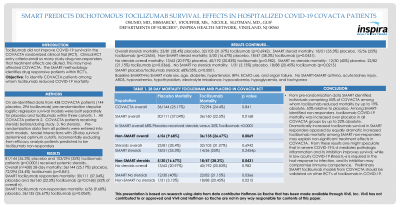
Results: 81/144 (56.3%) placebo and 103/294 (35%) tocilizumab patients (p < 0.0001) received systemic steroids.
Overall (n=438) 28-day mortality: 36/144 (25.17%) placebo, 72/294 (24.4%) tocilizumab (p=0.841). SMART tocilizumab responders mortality: 30/111 (37.04%) placebo and 36/160 (22.5%) tocilizumab (p=0.0168) (55% of overall n). SMART tocilizumab non-responders mortality: 6/56 (9.68%) placebo, 36/135 (26.67%) tocilizumab (p=0.0069).
Overall steroids mortality: 23/81 (28.4%) placebo, 32/103 (31.07%) tocilizumab (p=0.6942). SMART steroid mortality: 18/51 (35.3%) placebo, 15/56 (25%) tocilizumab (p=0.2454). Non-SMART steroid mortality: 5/30 (16.67%) placebo, 18/47 (38.3%) tocilizumab (p=0.0431).
No steroids overall mortality: 13/62 (20.97%) placebo, 40/192 (20.83%) tocilizumab (p=0.982). SMART no steroids mortality: 12/30 (40%) placebo, 22/82 (21.15%) tocilizumab (p=0.0366). No SMART no steroids mortality: 1/31 (3.13%) placebo, 18/88 (20.45%) tocilizumab (p=0.0215)
SMART placebo/tocilizumab steroids: 68%/35%, p< 0.0001.
Baseline SMART>No SMART male sex, age, diabetes, hypertension, BPH, ECMO use, and organ failure. NoSMART>SMART asthma, acute kidney injury, ARDS, hyponatremia, hypothyroidism, electrolyte imbalance, hypocalcemia, hypoglycemia, and tachypnea
Conclusion: From pre-randomization data SMART identified individuals comprising 55% of COVACTA among whom tocilizumab reduced mortality by up to 19% absolute, and 53% relative to placebo. Among SMART-identified non-responders, tocilizumab COVID-19 mortality was increased over placebo in all COVACTA groups by up to 22% absolute. Dramatically increased tocilizumab survival in SMART responders opposed by equally dramatic increased tocilizumab mortality among SMART non-responders may explain non-significant treatment effects in COVACTA. From these results, one might speculate that in severe COVID-19 IL-6 mediates pathologic inflammation and its inhibition improves survival, while in low acuity COVID-19 illness IL-6 is required in the host response to infection, and its inhibition may compromise immune competence. Preliminary SMART tocilizumab models from COVACTA should be validated on other RCT’s of tocilizumab in COVID-19
Background: Tocilizumab did not improve COVID-19 survival in the COVACTA randomized clinical trial (RCT). Clinical RCT entry criteria enroll so many study drug non-responders that treatment effects are diluted. This may have affected COVACTA. The SMART methodology identifies drug-responsive patients within RCT’s
Objective: To identify COVACTA patients among whom tocilizumab reduced COVID-19 mortality
Methods: On de-identified data from 438 COVACTA patients (144 placebo, 294 tocilizumab) pre-randomization stepwise logistic regression survival models were built separately for placebo and tocilizumab within three cohorts: 1. All COVACTA patients 2. COVACTA patients receiving steroids before/during the study. 3. No steroids. Pre-randomization data from all patients were entered into both models. Model interactions with 28-day survival determined optimum cutoffs, incrementally excluding from efficacy analysis patients predicted to be tocilizumab non-responders
Results: 81/144 (56.3%) placebo and 103/294 (35%) tocilizumab patients (p < 0.0001) received systemic steroids.
Overall (n=438) 28-day mortality: 36/144 (25.17%) placebo, 72/294 (24.4%) tocilizumab (p=0.841). SMART tocilizumab responders mortality: 30/111 (37.04%) placebo and 36/160 (22.5%) tocilizumab (p=0.0168) (55% of overall n). SMART tocilizumab non-responders mortality: 6/56 (9.68%) placebo, 36/135 (26.67%) tocilizumab (p=0.0069).
Overall steroids mortality: 23/81 (28.4%) placebo, 32/103 (31.07%) tocilizumab (p=0.6942). SMART steroid mortality: 18/51 (35.3%) placebo, 15/56 (25%) tocilizumab (p=0.2454). Non-SMART steroid mortality: 5/30 (16.67%) placebo, 18/47 (38.3%) tocilizumab (p=0.0431).
No steroids overall mortality: 13/62 (20.97%) placebo, 40/192 (20.83%) tocilizumab (p=0.982). SMART no steroids mortality: 12/30 (40%) placebo, 22/82 (21.15%) tocilizumab (p=0.0366). No SMART no steroids mortality: 1/31 (3.13%) placebo, 18/88 (20.45%) tocilizumab (p=0.0215)
SMART placebo/tocilizumab steroids: 68%/35%, p< 0.0001.
Baseline SMART>No SMART male sex, age, diabetes, hypertension, BPH, ECMO use, and organ failure. NoSMART>SMART asthma, acute kidney injury, ARDS, hyponatremia, hypothyroidism, electrolyte imbalance, hypocalcemia, hypoglycemia, and tachypnea
Conclusion: From pre-randomization data SMART identified individuals comprising 55% of COVACTA among whom tocilizumab reduced mortality by up to 19% absolute, and 53% relative to placebo. Among SMART-identified non-responders, tocilizumab COVID-19 mortality was increased over placebo in all COVACTA groups by up to 22% absolute. Dramatically increased tocilizumab survival in SMART responders opposed by equally dramatic increased tocilizumab mortality among SMART non-responders may explain non-significant treatment effects in COVACTA. From these results, one might speculate that in severe COVID-19 IL-6 mediates pathologic inflammation and its inhibition improves survival, while in low acuity COVID-19 illness IL-6 is required in the host response to infection, and its inhibition may compromise immune competence. Preliminary SMART tocilizumab models from COVACTA should be validated on other RCT’s of tocilizumab in COVID-19

